Current & Emerging Treatments
Understanding the shared pathophysiology of type 2 inflammatory (T2I) conditions helps explain the clinical success of targeted therapies across multiple conditions and supports a unified treatment approach for patients with T2I disease comorbidities.1,2 Treatment of T2I diseases has evolved significantly in recent years, with targeted therapies offering new opportunities to optimize care and reduce adverse events.
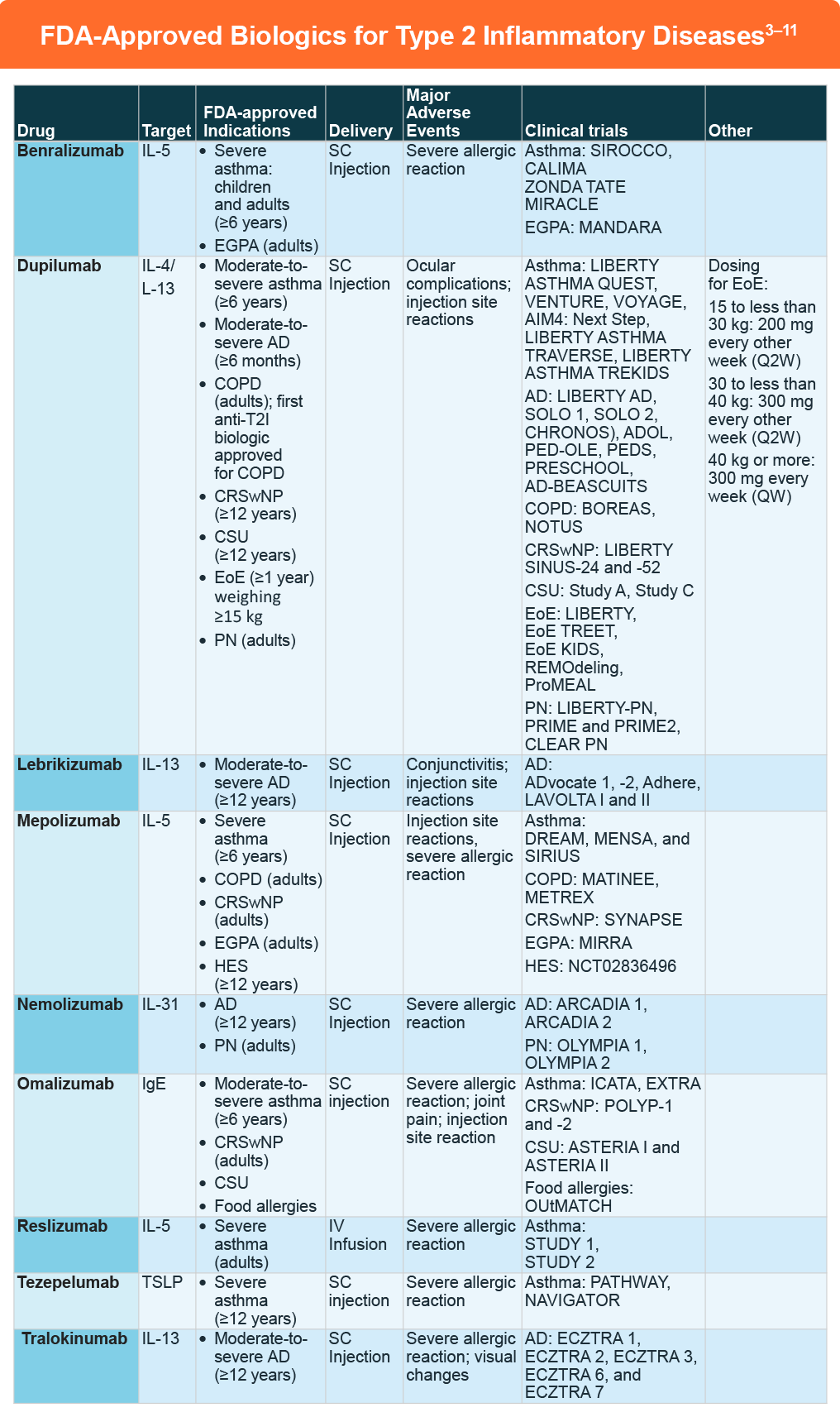
The numerous clinical trials for these compounds in T2I diseases is beyond the scope of this content. However, a few recent studies are worth highlighting:
- Dupilumab was FDA approved in September 2024 as an add-on maintenance treatment for adult patients with inadequately controlled COPD and an eosinophilic phenotype based on the BOREAS and NOTUS clinical trials. It is the first anti-T2I biologic to be approved for the condition.In the phase 3 BOREAS trial (N=939), COPD patients with blood eosinophil count of at least 300/mL and elevated exacerbation risk received dupilumab or placebo every 2 weeks.12 The annualized rate of moderate or severe exacerbations was lower for dupilumab versus placebo (rate ratio: 0.70, 95% CI: 0.58-0.86; P < .001). Significant improvements with dupilumab were also noted for pre-bronchodilator FEV1 that was maintained through to week 52, as well as St. George’s Respiratory Questionnaire and ERS-COPD scores at week 52. Adverse events were similar between dupilumab and placebo. In the replicate phase 3 NOTUS trial, dupilumab was also associated with fewer exacerbations and better lung function compared to placebo.13
- Lebrikizumab was FDA approved in September 2024 for the treatment of adults and pediatric patients 12 years of age and older who weigh at least 40 kg with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. The approval was based on the outcomes for the ADvocate 1, ADvocate 2, and ADhere studies. The former two demonstrated significant improvements in IGA scores 0-1 and EASI-75 response at 16 weeks for AD patients treated with lebrikizumab monotherapy although there were higher rates of conjunctivitis in the lebrikizumab group.14 These findings were consistent with the 16-week findings in the ADhere study that assessed lebrikizumab in combination with TCS.15
- Nemolizumab was FDA approved in September 2024 for the treatment of adults with PN based on the outcomes of the OLYMPIA 1 and OLYMPIA 2 trials. In OLYMPIA 2 (N=274), itch responses at week 16 were 56.3% versus 20.9% for nemolizumab versus placebo, respectively (37.4 percentage point difference, 95% CI: 26.3-48.5), while PP-NRS scores of less than 2 were 35.0% versus 7.7%.16 At Week 16 in the OLYMPIA 1 trial, patients receiving nemolizumab versus placebo similarly achieved a greater ≥4- point improvement in PP NRS (58.4% vs 16.7%; P< .0001).17
Investigational Therapies:
There are several therapies under investigation for type 2-mediated diseases, including:
- Depemokimab. This long-acting IL-5 inhibitor is in late-stage clinical trials for eosinophilic granulomatosis with polyangiitis (OCEAN), CRSwNP (ANCHOR), and hypereosinophilic syndrome (DESTINY).18,19
- Itepekimab. This IL-33 inhibitor is being studied for COPD (AERIFY-1 and AERIFY-2).21
- Tezepelumab. this TSLP inhibitor is being studied for eosinophilic esophagitis in the phase 3 CROSSING trial25 and for COPD26 in the COURSE study:
References
- Gieseck RL, 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62-76.
- Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15:35-50.
- Tralokinumab (ADBRY®). PI 2025 (https://mc-df05ef79-e68e-4c65-8ea2-953494-cdn-endpoint.azureedge.net/-/media/corporatecommunications/us/therapeutic-expertise/our-product/adbrypi.pdf?rev=d8ced7cbd6874a6997427ab88a2093e0#page=19).
- Reslizumab (CINQAIR®). PI 2020 (https://www.cinqair.com/globalassets/cinqair/prescribinginformation.pdf).
- Dupilumab (DUPIXENT®). Prescribing information (PI). 2025 (https://www.regeneron.com/downloads/dupixent_fpi.pdf).
- Lebrikizumab-lbkz (EBGLYSSTMTM). PI 2025 (https://uspl.lilly.com/ebglyss/ebglyss.html#pi).
- Benralizumab (FASENRA®). PI 2024
(https://www.azpicentral.com/fasenra/fasenra.pdf) - Nemolizumab (NEMLUVIO®). PI 2025
(https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/761391s001s002lbl.pdf) - Mepolizumab (NUCALA®). PI 2025 (https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL-IFU-COMBINED.PDF).
- Tezepelumab (TEZSPIRE®). PI 2025 (https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/e306dc06-d580-4457-b15f-9f28545ad63a/e306dc06-d580-4457-b15f-9f28545ad63a_viewable_rendition__v.pdf).
- Omalizumab (XOLAIR®). PI 2024 (https://www.gene.com/download/pdf/xolair_prescribing.pdf).
- Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab for COPD with blood eosinophil evidence of type 2 inflammation. N Engl J Med. 2024;390:2274-2283.
- Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab reduces exacerbations and improves lung function in patients with chronic obstructive pulmonary disease and emphysema: Phase 3 randomized trial (BOREAS). Respir Med. 2025;236:107846.
- Silverberg JI, Guttman-Yassky E, Thaçi D, et al. Two phase 3 trials of lebrikizumab for moderate-to-severe atopic dermatitis. N Engl J Med. 2023;388:1080-1091.
- Simpson EL, Gooderham M, Wollenberg A, et al. Efficacy and safety of lebrikizumab in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: A randomized clinical trial (ADhere). JAMA Dermatol. 2023;159:182-191.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Ständer S, Yosipovitch G, Legat FJ, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020;382:706-716.
- Jackson David J, Wechsler Michael E, Jackson Daniel J, et al. Twice-yearly depemokimab in severe asthma with an eosinophilic phenotype. N Engl J Med. 2024;391:2337-2349.
- Waldron J. GSK’s depemokimab reduces polyps in phase 3 trials, adding weight to upcoming filing. October 14, 2024. https://www.fiercebiotech.com/biotech/gsks-depemokimab-reduces-polyps-phase-3-trials-adding-weight-upcoming-filing
- Efficacy and Safety of Dupilumab in Patients With Bullous Pemphigoid: Results From LIBERTY-BP ADEPT Phase 2/3 Study. Late Breaking Research Session 2. Presented at 2025 American Academy of Dermatology Annual Meeting; March 8, 2025; Orlando, Florida.
- Casale, Thomas et al. Dupilumab Improves Itch And Urticaria Activity In Patients With Chronic Spontaneous Urticaria: Pooled Results From Two Phase 3 Trials (LIBERTY-CSU CUPID Study A and Study C). JAACI. 2024; 155 (2):AB428
- Zhao Y, Zhang L, Wu L, et al. Long-term efficacy and safety of stapokibart for moderate-to-severe atopic dermatitis: 52-week results from a phase 3 trial. Allergy. 2024;00:1-10.
- Keymed Biosciences Announces Interim Results for First Half of 2024. August 28, 2024. https://www.prnewswire.com/news-releases/keymed-biosciences-announces-interim-results-for-first-half-of-2024-302232637.html
- Conrad C, Schlapbach C. Prurigo nodularis forecast: Light type 2 inflammation with high chances of fibrosis. J Allergy Clin Immunol. 2024;153:93-94.
- Sharlin CS, Collins MH, Bolton SM, et al. Induction of sustained remission and reversal of pathologic transcriptome achieved with tezepelumab in an adolescent with eosinophilic esophagitis. J Allergy Clin Immunol Pract. 2024;12(11):3147-3149.e2. doi: 10.1016/j.jaip.2024.08.013)
- Singh D, Brightling CE, Rabe KF, et al. Efficacy and safety of tezepelumab versus placebo in adults with moderate to very severe chronic obstructive pulmonary disease (COURSE): a randomised, placebo-controlled, phase 2a trial. Lancet Respir Med. 2025 Jan;13(1):47-58. doi: 10.1016/S2213-2600(24)00324-2
All URLs accessed January 7, 2026

